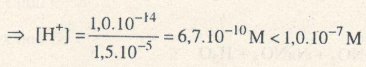2. Chất chỉ thị axit – bazơ
- Là chất có màu biến đổi phụ thuộc vào giá trị pH của dung dịch.
- Dựa vào sự chuyển màu của giấy quỳ và dung dịch phenolphtalein xác định được môi trường của dung dịch, dựa vào màu của giấy chỉ thị vạn năng có thể xác định được gần đúng giá tri pH của dung dịch.
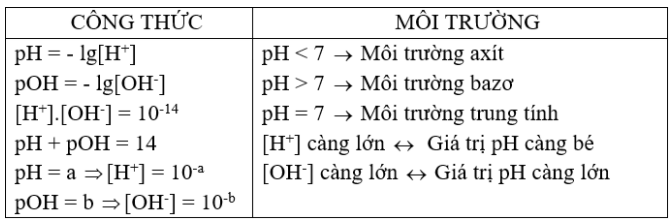
Ta có: Nếu [H+] = 1,0.10–a M thì pH = a.
=> pH = -lg [H+]
Ví dụ : [H+] = 10-3M pH = 3 : Môi trường axit.
pH + pOH = 14
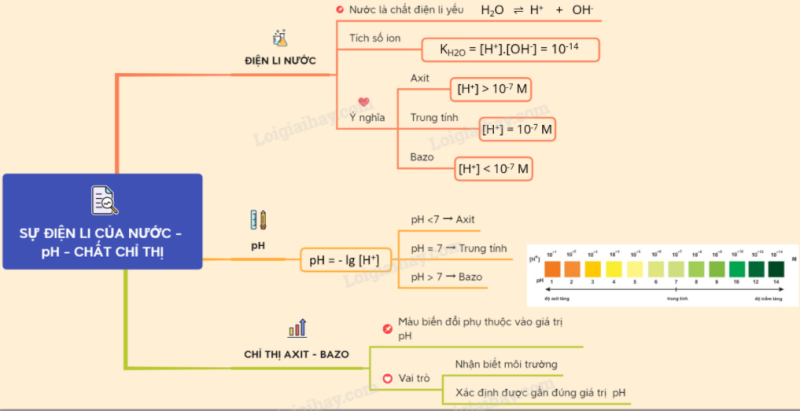
Sơ đồ tư duy: Sự điện li của nước. pH. Chất chỉ thị Axit - bazơ
Phương pháp giải một số dạng bài tập về sự điện li của nước, pH
Dạng 1: Lý thuyết về sự điện li của nước và pH
* Một số lưu ý cần nhớ:
|
- Nước là chất điện li rất yếu : H2O H+ + OH-
Tích số ion của nước: = [H+][OH-] =10-14 M (đo ở 25oC)
Môi trường axit :
[H+] > [OH–] hay [H+] > 1,0.10–7M => pH < 7
Môi trường kiềm :
[H+] < [OH–] hay [H+] < 1,0.10–7M. => pH > 7
Môi trường trung tính :
[H+] = [OH–] = 1,0.10–7M. => pH = 7
|
* Một số ví dụ điển hình:
Ví dụ 1: Cho các muối sau đây : NaNO3 ; K2CO3 ; CuSO4 ; FeCl3 ; AlCl3 ; KCl. Các dung dịch có pH = 7 là :
A. NaNO3 ; KCl.
B. K2CO3 ; CuSO4 ; KCl.
C. CuSO4 ; FeCl3 ; AlCl3.
D. NaNO3 ; K2CO3 ; CuSO4.
Hướng dẫn giải chi tiết:
- Dung dịch có môi trường trung tính sẽ có p H = 7
Mặt khác muối có môi trường trung tính là muối của KL mạnh và gốc axit mạnh
Đáp án A
Ví dụ 2: Trong số các dung dịch : Na2CO3, KCl, CH3COONa, NH4Cl, NaHSO4, C6H5ONa, những dung dịch có pH > 7 là :
A. Na2CO3, NH4Cl, KCl.
B. Na2CO3, C6H5ONa, CH3COONa.
C. NH4Cl, CH3COONa, NaHSO4.
D. KCl, C6H5ONa, CH3COONa.
Hướng dẫn giải chi tiết:
Dung dịch có p H lớn hơn 7 là dung dịch khi thủy phân trong nước cho môi trường bazo
Mặt khác muối có môi trường bazo là muối của KL mạnh và gốc axit yếu.
A loại do KCl có môi trường trung tính
C loại do NaHSO4 có môi trường axit
D loại do KCl có môi trường trung tính.
Đáp án B.
Ví dụ 3: Cho phản ứng :
2NO2 + 2NaOH → NaNO2 + NaNO3 + H2O
Hấp thụ hết x mol NO2 vào dung dịch chứa x mol NaOH thì dung dịch thu được có giá trị
A. pH = 7. B. pH > 7.
C. pH = 0. D. pH < 7.
Hướng dẫn giải chi tiết:
n NO2 = n NaOH
=> 2 chất trên phản ứng vừa đủ
Mặt khác, sau phản ứng sinh ra NaNO3 có môi trường trung tính và NaNO2 có môi trường bazo (do là muối của KL mạnh và axit yếu)
=> Sau phản ứng dung dịch thu được có môi trường bazo => pH > 7
Đáp án B.
Dạng 2: Xác định pH của axit, bazo mạnh
* Một số lưu ý cần nhớ:
|
- Tính số mol H+, OH- có trong dung dịch
- Nồng độ H+, OH- có trong dung dịch => pH
|
* Một số ví dụ điển hình:
Ví dụ 1: Trộn 10g dung dịch HCl 7,3% với 20g dung dịch H2SO4 4,9% rồi thêm nước để được 100ml dung dịch A. Tính pH của dung dịch A.
Hướng dẫn giải chi tiết:
m HCl = 10 . 7,3% = 0,73 gam
=> n HCl = 0,73 : 36,5 = 0,02 mol
m H2SO4 = 20 . 4,9% = 0,98 gam
=> n H2SO4 = 0,98 : 98 = 0,01 mol
Ta có phương trình điện li như sau:
HCl →H+ + Cl-
0,02 0,02
H2SO4 → 2H+ + SO42-
0,01 0,02
=> n H+ = 0,02 + 0,02 = 0,04 (mol)
V dung dịch sau khi pha trộn là 100ml = 0,1 lít
=> [H+] = 0,04 : 0,1 = 0,4M
=> p H = -log[H+] = 0,4
Ví dụ 2: Hoà tan m gam Zn vào 200 ml dung dịch H2SO4 0,4M thu được 1,568 lít khí hiđro và dung dịch X. Tính pH của dung dịch X?
Hướng dẫn giải chi tiết:
n H2SO4 = 0,4 . 0,2 = 0,08 (mol)
n H2 = 1,568 : 22,4 = 0,07 (mol)
Ta có phương trình hóa học:
Zn + H2SO4 → ZnSO4 + H2 (1)
(1) => n H2SO4 phản ứng = n H2 = 0,07 mol
=> n H2SO4 dư = 0,08 – 0,07 = 0,01 mol
Ta có phương trình điện li:
H2SO4 → 2H+ + SO42-
0,01 0,02
=> [H+] = 0,02 : 0,2 = 0,1
=> pH = 1
Dạng 3: Xác định pH của axit , bazo yếu
* Một số lưu ý cần nhớ:
|
- Viết phương trình điện li
- Dựa vào dữ kiện đề bài áp dụng công thức tính độ điện li và hằng số điện li axit, bazo Ka, Kb
Công thức tính độ điện li:
C: nồng độ chất điện li; Co nồng độ chất tan
Ta có phương trình điện li của axit;
HA A- + H+
=> Ka chỉ phụ thuộc vào bản chất của axit và nhiệt độ
Ta có phương trình điện li của bazo:
BOH B+ + OH-
=> Kb chỉ phụ thuộc vào bản chất của bazo và nhiệt độ.
Ngoài ra, ta có thể áp dụng được công thức tính nhanh như sau:
|
Một số ví dụ điển hình:
Ví dụ 1: Giá trị pH của dung dịch axit fomic 1M (Ka = 1,77.10-4) là :
A. 1,4. B. 1,1.
C. 1,68. D. 1,88.
Hướng dẫn giải chi tiết:
Phương trình điện li :
HCOOH HCOO-+ H+ (1);
bđ: 1
p.li a.1 a.1 a.1
cb: 1– a a a
Tại thời điểm cân bằng ta có :
(2)
=>
Theo (1) [H+] = a = 0,0132MpH = -lg[H+] = 1,88.
Đáp án D.
Ví dụ 2: Dung dịch CH3COONa 0,1M (Kb = 5,71.10-10) có [H+] là :
A. 7,56.10-6 M.
B. 1,32.10-9 M.
C. 6,57.10-6 M.
D. 2,31.10-9 M.
Hướng dẫn giải chi tiết:
Phương trình điện li :
CH3COONa → CH3COO- + Na+
CM : 0,1 → 0,1
Phương trình phản ứng thủy phân :
CH3COO-+H2O CH3COOH+ OH-
CM : a.0,1 → a . 0,1
Ta có:
Sử dụng công thức ta có :
Dạng 4: Bài toán pha loãng dung dịch axit, bazo.
* Một số lưu ý cần nhớ
|
- Viết phương trình điện li
- Từ dữ kiện đề bài, tính lại thể tích dung dịch lúc sau
=> Thể tích nước cần thêm vào để thỏa mãn đề bài
|
* Một số ví dụ điển hình:
Ví dụ 1: Phải thêm bao nhiêu ml dung dịch HCl 1M vào 90 ml nước để được dung dịch có pH = 1?
Hướng dẫn giải chi tiết:
pH = 1 => [H+] = 0,1M
Đặt thể tích dung dịch HCl 1M cần thêm vào là V (lít)
=> nHCl = V mol
Sau khi trộn với 90 ml H2O:
[H+] = CM HCl sau trộn = = 0,1M
=> V = 0,01 lít = 10 ml
Ví dụ 2: Dung dịch HCl có pH=3. Hỏi phải pha loãng dung dịch HCl đó bằng nước bao nhiêu lần để được dung dịch HCl có pH = 4. Giải thích?
Hướng dẫn giải chi tiết:
Giả sử dung dịch HCl ban đầu có thể tích V1 có pH = 3 => [H+] = 10-3
Số mol H+ ban đầu là :V1.10-3 mol (1)
Gỉa sử thể tích H2O cần thêm vào là V2
Số mol H+ trong dung dịch pH= 4 là (V1 + V2 ).10-4 (2)
Việc pha loãng dung dịch chỉ làm thay đổi nồng độ mol/l chứ không làm thay đổi số mol H+.
Vì vậy : (V1 + V2 ).10-4 = V1.10-3
=> 9 V1 = V2
Vậy phải pha loãng dung dịch gấp 10 lần (nước thêm vào gấp 9 lần thể tích ban đầu)